In this article, we will share to you a technique on how ABG interpretation is done and it’s made easy as if you are drinking your Creamy Coffee.
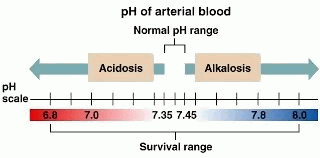
Arterial Blood Gas more commonly known as ABG, is one of the most routinely taken lab test to our patients and there’s a very good reason why – because the results gathered from this test may tell you a very significant information about your patient’s condition. ABG measures the amount of oxygen and carbon dioxide in our blood; an imbalance to these factors may lead to the increase or decrease of our blood’s pH levels – which is referred to as Acidemia or Alkalemia.
Taxi and Its passengers
ABG results give you vital information about the severity of your patient’s condition, but it may also give an indication of how your patient is coping to treatment; that is the reason why this test is commonly done on a routine basis. Now, you may wonder, how is this test taking a very broad scope of our body’s condition? Well, the answer is simple; because blood circulates all over your body. To give light to the basics of the blood circulation, think of blood cells, particularly the Red Blood Cells (RBCs) as a Taxi. From the heart, it carries its passengers which is the Oxygen into the various parts of our body, mainly the organs through a highway of Arteries. Now, once the Taxi (RBCs) arrives at the destination (Organs), it then drops its passengers (Oxygen) through a process called Diffusion – wherein the Taxi takes in new passengers, the Carbon Dioxide (CO2). As the taxi goes back to its terminal (Heart), it goes through a different highway called the veins, and then passes thru the Vena Cava so that the Taxi can take its new passengers to the Lungs in order to go through another process of Diffusion. And the cycle goes on.
As we discussed about the circulation of oxygen and carbon dioxide in our body, the question lies behind the reason why an imbalance with the normal amount of Oxygen and/or Carbon Dioxide. Say, for example, if a patient has a difficulty of breathing, there must be an underlying reason why the patient is going through this state. Symptoms such as the one mentioned above is one of the characteristics of asthma or other pulmonary diseases. Nonetheless, diseases like diabetes, or kidney or heart failure can also lead to an imbalance of the blood gas.
Respiratory | Renal Regulation of Acid-Base
Our body has two ways to regulate it’s Acid and Base balance. The respiratory and the Renal Regulation. Let’s try to make this as easy as we can possibly tackle, you ready?

Respiratory Regulation is the body’s way to maintain Acid-Base Homeostasis by the use of the Respiratory System to take in oxygen from the environment (inhalation) and eliminating carbon dioxide to the environment (exhalation). The main concern here that you need to take into consideration is that Carbon Dioxide is ACIDIC by nature. means the higher the CO2 there is in the body, specifically in the blood, the more acidic your blood pH will get. So, CO2 is ACIDIC. When you see the letters in the lab results and there is CO2 written there, you always think that CO2 is ACIDIC – keep that in mind, you’re gonna need it later.
Renal Regulation is the body’s way of maintaining Acid-Base balance thru the use of the Renal System. Now, your ultimate take in Renal Regulation is the Bicarbonate (HCO-3), and that it controls the metabolic component of the body’s buffer system. Bicarbonate is a buffer and its levels are controlled by the renal system. This means that it keeps the blood from being too acidic or it maintains the equilibrium of the Acid-Base balance in your bloodstream.
The Coffee & The Creamer
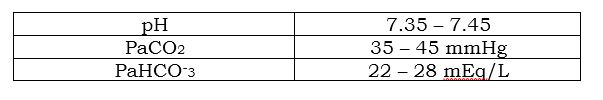
This is where everything becomes easy. Before you get excited about learning the principle behind the coffee and the creamer, let us first establish some normal VALUES THAT WILL BE USED to interpret your ABG with ease.
Think of it in this manner, when you drink coffee, sometimes when you put too much coffee in your cup, it becomes very bitter and since coffee is acidic by nature, you can sometimes taste the sourness in your cup if your coffee is too much. On the other hand, in order for you to compensate for the bitterness and sourness and try to balance the taste of it, tendencies are, you add a creamer.
The creamer then neutralizes the bitterness and sourness and it makes you enjoy your coffee as you would’ve wanted it to be – just the right balance. That is the relationship of pH, CO2, and HCO-3, the pH is the hot water, CO2 as the coffee and HCO-3 as the creamer – and all we need to do is to balance that coffee and creamer in order for us to enjoy our cup. In the same objective, our body also has that ability to efficiently balance its coffee and creamer inside. In this table, you can see that I am giving you only 3 values, the pH, CO2, and HCO-3. The reason for it is for you to concentrate and be commonly acquainted with the terms and what your doctor or senior nurses mean when you hear that “the pH and the CO2 are both moving at the same way, that is Acidosis”. I want to provide another table so that we can see things from a better perspective.
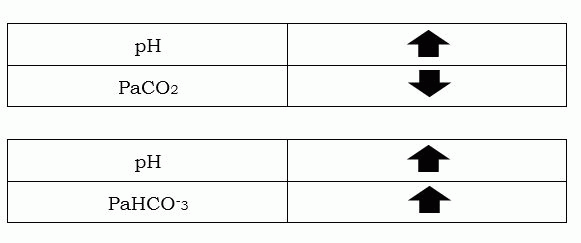
Respiratory Opposite | Metabolic Equal (R.O.M.E.)
This is the ROME principle; it uses arrows for you to easily distinguish the acid-base imbalance if it is Respiratory or Metabolic. How it is done is very simple; refer to the table above as I explain it in detail:
Respiratory Imbalance
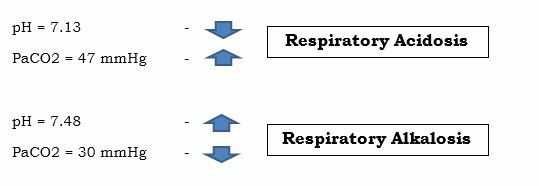
If the arrows of both pH and CO2 ARE pointing in the opposite direction, you can say that the acid-base balance is respiratory. This is actually what you constantly hear in the work field “Both the pH and the CO2 are moving on the same way.” – the “same way” means that they are either moving towards acidosis or alkalosis. The common misinterpretation is that we often forget, that when the pH is high, it yields alkalosis, and if it is low, it’s an acidosis. Taking this to consideration, when is CO2 high, it yields acidosis, and if it’s low, it is alkalosis. There you go, pH and CO2 move towards the same way, but in a different direction. Thus, Respiratory Opposite.
Metabolic Imbalance
When you look at the table above, and you will notice that as the pH and the go HCO-3 up, THEY BOTH AGREE on yielding an alkalosis.On the other hand, if both go down, it will give us an acidosis. Unlike in the relationship of pH and CO2, this HCO-3 is a buffer that is there to safeguard your body from having these imbalances. But there are certain conditions in which this particular buffer system HCO-3 causes the problem and the CO2 becomes the compensator. So, to recap it, when both pH and HCO-3 go up, it is alkalosis; when both go down, it is acidic – coming up to the phrase Metabolic Equal.
ABG interpretation made easy
Steps to Interpret ABG
1. Check the pH (7.35 – 7.45) – in this part you are going to figure out if it’s acidosis (below 7.35) or alkalosis (above 7.45).
2. Check the PaCO2 – to rule out if it’s respiratory or metabolic (Respiratory Opposite). In this step, take note where your pH is pointing, is it above 7.45 or below 7.35; and then, below it writes the value of your PaCO2, and then draw an arrow also. The arrows will give you a very clear view, if the arrows are pointing at the same or the opposite direction. Then you will easily identify that it is Respiratory or not.
Here are some examples:
Practice Quiz:
- pH = 7.31 – Metabolic Acidosis
- PaCO2 = 32 mmHg –
- pH = 7.50 – Metabolic Alkalosis
- PaCO2 = 47 mmHg –
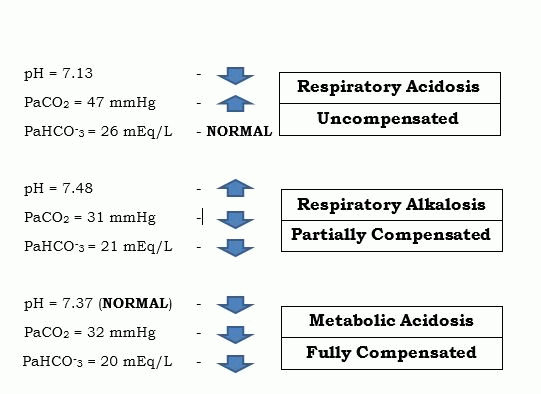
3. Check the compensation – in this part, we will now see if the result you have is either Uncompensated, Partially Compensated or Fully Compensated. For the compensation, it’s best to practice tic-tac-toe method but if you want to stick with one principle which is R.O.M.E., it is also possible. The first thing that you need to keep in mind is the range value of the pH (7.35 – 7.45). Looking at that range, there is a sweet spot of 7.40 – keep that in mind. Next, is to incorporate now the creamer in our coffee. Let’s see some examples:
Let’s analyze these results. For the first example, it is “Uncompensated” when there is an imbalance happening in your body, and the PaHCO-3 FAILED to do its job, which is to compensate. As we observe, the PaHCO-3 did not increase its value to compensate with the acidosis, it just remained within its normal range.
In the second example, we said that it was a “Partially Compensated” when the buffer did its job but the pH still didn’t go back to its normal range. As we can observe there, the PaCO2 was low indicating an alkalosis so the PaHCO-3 levels went down, in order to balance the alkalinity but still the pH is abnormal – that’s what you call a partial compensation.
The third example shows a “Fully Compensated” Metabolic Acidosis. Now, when can we say, “fully compensated” and why is the arrow still pointing downwards when the pH level shows NORMAL (7.37)? Remember the sweet spot of pH (7.40)? Yes, this is where it plays its role. The pH is in the normal range but the result of 7.37 is below the sweet spot of 7.40, hence we are more likely to agree that it is clinging more on the acidic side. Since the PaCO2 was low, we decided that it is a Metabolic Acidosis, and when we checked the PaHCO-3, it was also low concluding that the result was really metabolic and the PaCO2 was the one trying to compensate by lowering its values. But even though the PaCO2 and the PaHCO-3 are abnormal, as long as the pH is in its normal range – it will yield a full compensation. We can say that since it clings more to the acidic side because it’s lower than 7.40, it must’ve come from acidosis before it normalized.
Practice Quiz:
- pH = 7.20 –
- PaCO2 = 32 mmHg – Metabolic Acidosis Partially Compensated
- PaHCO–3 = 20 mEq/L –
- pH = 7.47 –
- PaCO2 = 49 mmHg – Respiratory Alkalosis Uncompensated
- PaHCO–3 = 26 mEq/L –

ALRIGHT! Isn’t that easy?! All you need to remember when reading ABG is the coffee and the creamer, R.O.M.E. and how to distinguish the three types of compensation. When you master these principles, you’ll be rocking the ABG results as easy as you’re taking a pulse rate, using an oximeter – it’s THAT easy. But you can’t expect yourself to be proficient with these principles in a blink of an eye, of course after knowing all these things, it’s now in your hands, and your brain to apply what you’ve learned. Take all those ABG results in your patients’ files and practice thru those. Only when you do things regularly will you be able to develop skills and expertise. I hope that you’ve learned a thing or two with ABGs. Stick around for more tricks and tips on what’s and how’s here at RNspeak.
“Knowledge is of no value if you don’t put it into practice.” ~Anton Chekhov





![Caring for Patients with Tracheostomy and Nursing Diagnoses [ Updates] tracheostomynursingprocedure](https://rnspeak.com/wp-content/uploads/2020/10/tracheostomynursingprocedure_725820712-238x178.jpg)




I was thinking the same thing.
pH = 7.47 –
PaCO2 = 49 mmHg – Respiratory Alkalosis Uncompensated
PaHCO–3 = 26 mEq/L
shouldn’t this be metabolic alkalosis uncompensated on your practice quiz?
pH and PaCO2 are both up so arrows are both pointing in the same direction?
exactly, im more confused now lol