The outbreak of Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has thus far killed over 5,00,000 people and infected over 10 million people worldwide, resulting in catastrophe for humans
This article attempts to provide a timely and comprehensive review of the emerging disease. We will cover the basics about the epidemiology, etiology, virology, diagnosis, treatment, prognosis, prevention and nursing considerations.
Introduction
Corona virus disease 2019 (COVID-19) is an infectious disease caused by a new strain of virus from corona virus family called severe acute respiratory syndrome corona virus 2 (SARS-CoV-2)
Cause
- Caused by severe acute respiratory syndrome corona virus 2 (SARS-CoV-2)
- First identified during an outbreak of atypical respiratory illness cases in Wuhan City, China.
- Corona viruses comprise a group of viruses that cause illness in humans and animals. Under the microscope the viruses look like they are covered with pointed structures that surround them like a corona, or crown and hence named.
- Rarely, some coronaviruses that typically infect animals can evolve and infect people and then spread between people. SARS-CoV-2 is likely one such virus, postulated to have originated in a seafood market in Wuhan city in Hubei Province of China in mid-December, 2019, has now spread to 214 countries/territories/areas worldwide.
- Severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) are also caused by coronaviruses transmitted from animals to humans. [/su_box]
Corona virus outbreak and pandemic
- The disease was initially reported to the WHO on December 31, 2019. On January 30, 2020, WHO declared the outbreak a “Public Health Emergency of International Concern”. Subsequently WHO declared the disease a pandemic on 11th March, 2020.
- As of July 1, 2020, the disease has been confirmed in over 10.7 million people worldwide and has resulted in more than 5,00,000 deaths. More than 180 countries have reported confirmed cases of COVID-19 other than Antarctica.
- In U S, more than 2.7 million cases of COVID-19 have been confirmed as of July 1, 2020, resulting in over 1,30,000 deaths. As of March 26, 2020, the United States has more confirmed cases of COVID-19 than any other country in the world, including China and Italy.
Disease Epidemiology
Cause
- SARS-CoV-2 is an enveloped RNA beta coronavirus related to the Severe Acute Respiratory Syndrome (SARS) virus.
- Like MERS and SARS, SARS-CoV-2 has a zoonotic source (originated in bats).
Source of infection
The persons infected by the novel coronavirus (SARS-CoV-2) are the main source of infection.
Route of Transmission
- Direct person-to-person transmission (through close contact, mainly through respiratory droplets that are released when the infected person sneezes, coughs or talks).
- These droplets may land on surfaces, where the virus remains viable. When a person touches an infected surface and then touches his or her eyes, nose, or mouth infection can occur.
- At present, contact with fomites is believed to be less significant than person-to-person spread as a means of transmission.
Incubation period
- The incubation period is about 2–14 days.
- The exact interval during which a person with COVID-19 is infectious is uncertain. As per the current evidence, the period of infectivity starts 2 days prior to onset of symptoms and may last up to 8 days.
- The duration of viral shedding varies significantly and may depend on severity of the disease.
- The extent and role played by pre-symptomatic/asymptomatic infections in transmission still remain under investigation.
According to the CDC, people at high risk of infection include persons in areas with ongoing local transmission, health workers caring for patients with COVID-19, close contacts of infected people, and travelers returning from places where reported local spread.
Pathophysiology
Most patients with COVID-19 have predominantly a respiratory tract infection associated with SARS-CoV-2 infection. However, in a small proportion of cases, they can progress to a more severe and systemic disease characterized by the Acute Respiratory Distress Syndrome (ARDS), sepsis and septic shock, multiorgan failure, including acute kidney injury (AKI) and cardiac injury.
Although details of cellular responses to this virus are not known, a likely course of events can be postulated based on past studies with SARS-CoV. Based on the cells that are likely infected, COVID-19 disease course can be divided into three phases that correspond to different clinical stages of the disease.
Stage 1: Asymptomatic state (initial 1–2 days of infection)
The inhaled virus SARS-CoV-2 likely binds to epithelial cells in the nasal cavity and starts replicating. ACE2 is the main receptor for both SARS-CoV2 and SARS-CoV. At this stage the virus can be detected by nasal swabs. Although the viral burden may be low, these individuals are infectious. The RT-PCR value for the viral RNA might be useful to predict the viral load and the subsequent infectivity and clinical course.
Stage 2: Upper airway and conducting airway response (next few days)
The virus propagates and migrates down the respiratory tract along the conducting airways, and a more robust innate immune response is triggered. Nasal swabs or sputum should yield the virus (SARS-CoV-2) as well as early markers of the innate immune response. At this time, the disease COVID-19 is clinically manifest.
For about 80% of the infected patients, the disease will be mild and mostly restricted to the upper and conducting airways
Stage 3: Hypoxia, ground glass infiltrates, and progression to ARDS
About 20% of the infected patients will progress to stage 3 and will develop pulmonary infiltrates and some of these will develop very severe disease. The virus now reaches the alveoli and infects alveolar type II cells. The virus propagates within type II cells, large number of viral particles are released, and the cells undergo apoptosis and die. The released viral particles infect type II cells in adjacent units and subsequently results in diffuse alveolar damage with fibrin rich hyaline membranes. The aberrant wound healing may lead to more severe scarring and fibrosis than other forms of ARDS.
SARS-CoV-2
⇓
Release inflammatory mediators (Proinflammatory cytokines interleukin IL-6, IL-10, granulocyte-colony stimulating factor (G-CSF), monocyte chemoattractant protein 1 (MCP1), macrophage inflammatory protein (MIP)1α, tumor necrosis factor (TNF)-α etc)
⇓
Endothelial damage of pulmonary vasculature, microvascular thrombosis and hemorrhage linked to extensive alveolar and interstitial inflammation
⇓
Vasculopathy, pulmonary intravascular coagulopathy, hypercoagulability, ventilation perfusion mismatch, and refractory ARDS. Hypoxemia, secondary to ARDS may also activate the coagulation cascade.
Prognosis
- Early reports shows COVID-19 is clinically milder than MERS or SARS in terms of severity and case fatality rate (the most recent CDC-estimated death rate has been around 0.4% for symptomatic cases).
- Deaths are more common in older people (>60 years) and in persons with serious underlying health conditions.
Case Definition
| Suspect |
| A. Patients with acute respiratory illness (fever and at least one sign/symptom of respiratory disease, e.g., cough, shortness of breath), and a history of travel to or residence in a location reporting community transmission of COVID-19 disease during the 14 days prior to symptom onset;
OR B. Patients with any acute respiratory illness and having been in contact with a confirmed or probable COVID-19 case in the last 14 days prior to symptom onset; OR C. Patients with severe acute respiratory illness (fever and at least one sign/symptom of respiratory disease, e.g., cough, shortness of breath; and requiring hospitalization) and in the absence of an alternative diagnosis that fully explains the clinical presentation. |
| Probable case |
| A. A suspect case for whom tests for the COVID-19 virus are inconclusive.
OR B. A suspect case for whom the tests could not be carriedout for any reason. |
| Confirmed case |
| A person with laboratory confirmation of COVID-19 infection, regardless of clinical signs and symptoms. |
*As per WHO surveillance guidelines[/su_table]
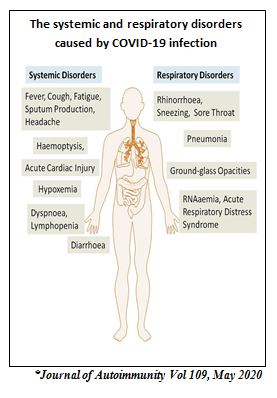
Clinical Presentation
Presentations of COVID-19 range from asymptomatic/mild symptoms to severe illness and mortality. Symptoms may develop 2 days to 2 weeks after exposure to the virus.
Cough and shortness of breath/difficulty in breathing plus at least two of the following symptoms may indicate COVID-19:
Other reported symptoms have included the following:
- Fatigue
- Sputum production
- Diarrhea
- Malaise
- Respiratory distress
Clinical Course
Illness Severity
Illness severity can range from mild to critical
- Mild to moderate (mild signs and symptoms up to mild pneumonia): 81%
- Severe (dyspnea, hypoxia, or more than 50% lung involvement on imaging): 14%
- Critical (respiratory failure, shock, or multiorgan dysfunction): 5%
Clinical Progression
Studies reports that among patients who developed severe disease, the medium time to dyspnea ranged from 5 to 8 days, the median time to acute respiratory distress syndrome (ARDS) ranged from 8 to 12 days, and the median time to Intensive care unit admission ranged from 10 to 12 days.
Complications
Reported complications of COVID-19 include:
- Pneumonia
- acute respiratory distress syndrome
- cardiac injury
- arrhythmia
- septic shock
- liver dysfunction
- acute kidney injury
- multi-organ failure
ARDS is a major complication in severe cases of COVID-19, affecting 20% – 41% of hospitalized patients.
Risk Factors
The major risk factors for severe disease are:
- Age more than 60 years
- Underlying non-communicable diseases (NCDs): diabetes, hypertension, cardiac disease, chronic lung disease, cerebro-vascular disease, chronic kidney disease, immune-suppression and cancer
Reinfection
There are no data on the possibility of re-infection with SARS-CoV-2 after recovery from COVID-19.
Diagnosis
Mild cases of COVID-19 may appear similar to the flu or a bad cold so diagnosis may be difficult with only a physical examination.
- The disease should be suspected in
-
- patients with respiratory tract symptoms and newly
- onset fever or
- in patients with severe lower respiratory tract symptoms with no clear cause.
-
- Suspicion increases if such patients have been in close contact with a person with confirmed or suspected COVID-19 in the preceding 14 days or have been in an area with community transmission.
The Infectious Diseases Society of America (IDSA) has issued testing recommendations in terms of tier-based priority groups.
| FIRST PRIORITY |
|
| SECOND PRIORITY |
| Symptomatic residents of long-term care and hospitalized patients not in the ICU. |
| THIRD PRIORITY |
| Those being treated in outpatient settings who meet criteria for influenza testing, including persons with certain comorbidities (eg, diabetes, COPD, CHF); pregnant women; and symptomatic pediatric patients with additional risk factors. |
| FOURTH PRIORITY |
| Persons who are undergoing monitoring for data collection and epidemiologic studies by health authorities. |
[/su_table]
Sample collection
Preferred sample
- Nasal and throat swab in viral transport media and transported in cold chain.
Alternate
- Nasopharyngeal swab, BAL/ endotracheal aspirate which has to be mixed with the viral transport medium and transported in cold chain.
General guidelines
- Use appropriate PPE for specimen collection (droplet and contact precautions for URT specimens; airborne precautions for LRT specimens).
- Maintain proper infection control when collecting specimens
- Restricted entry to visitors or attendants during sample collection
- Complete the requisition form for each specimen submitted
- Proper disposal of all waste generated
Diagnostic Laboratory Studies
- Polymerase chain reaction: Real time or Conventional RT-PCR test is recommended for diagnosis.
- Antibody testing: SARS-CoV-2 antibody tests are not recommended for diagnosis of current infection with COVID-19.
- Blood culture: For COVID-19 patients with severe disease, also collect blood cultures, ideally prior to initiation of antimicrobial therapy
- Laboratory findings: Lymphopenia is the most common lab finding (found in upto 83% of hospitalized patients). Lymphopenia, neutrophilia, elevated lactate dehydrogenase, elevated SGOT/SGPT levels, raised CRP, and high ferritin levels may be associated with greater illness severity. Elevated D-dimer have been associated with mortality. Procalcitonin usually normal on admission, may increase among those admitted to the ICU. Critical illness patients had high plasma levels of inflammatory makers, suggesting potential immune dysregulation.
- CT Scanning: Chest CT images from patients with COVID-19 typically demonstrate bilateral, peripheral ground glass opacities. Pleural effusion, pleural thickening, and lymphadenopathy have also been reported, although with less frequency.
- Chest Radiography: typically demonstrate bilateral air-space consolidation and ground-glass opacities, though patients may have unremarkable chest radiographs early in the disease.
MANAGEMENT
- There is no specific treatment for the virus at present.
- No vaccine is currently available for SARS-CoV-2 and avoidance is the principal method of deterrence.
- Numerous collaborative efforts to discover and evaluate effectiveness of vaccines, antivirals, immunotherapies, and monoclonal antibodies have rapidly emerged.
Prevention
No vaccine is currently available for SARS-CoV-2 and avoidance is the principal method of deterrence.
General measures for prevention of viral respiratory infections include the following:
- Handwashing with soap and water for at least 20 seconds. An alcohol-based hand sanitizer may be used if soap and water are unavailable.
- Avoid touching eyes, nose, and mouth with unwashed hands.
- Maintain social distancing: Individuals should avoid close contact with sick people. Maintain at least 1 metre distance with anyone who is coughing or sneezing. Sick people should stay at home.
- Follow good respiratory hygiene: Coughs and sneezes should be covered with a tissue, followed by disposal of the tissue in the trash.
- Proper disinfection: Frequently touched objects and surfaces should be cleaned and disinfected regularly.
- Seek medical advice promptly: If any symptoms develop seek medical advice promptly.
Preventing/minimizing community spread of COVID-19
The CDC has recommended the below measures to prevent/control community spread.
- The people in areas with prevalent COVID-19 should be vigilant for potential symptoms of infection and should stay home as much as possible, should practice social distancing (maintaining a distance of 6 feet from other persons) when leaving home is necessary.
- Individuals with increased risk for infection such as: (1) those who have had close contact with a person with known or suspected COVID-19 or (2) international travelers should observe increased precautions. These include (1) self-quarantine for at least 2 weeks (14 days) from the time of the last exposure and distancing (6 feet) from other persons at all times and (2) self-monitoring for cough, fever, or dyspnea with temperature checks twice a day.
- The general public, should begin wearing face coverings in public settings where social-distancing measures are difficult to maintain in order to abate the spread of COVID-19.
Clinical Management and Treatment
Prophylaxis
Pre-Exposure Prophylaxis
- At present, no agent is known to be effective in preventing SARS-CoV-2 infection before exposure. Clinical trials using hydroxychloroquine, chloroquine, or HIV protease inhibitors are in underway.
Post-Exposure Prophylaxis
- At present, no agent is known to be effective for preventing SARS-CoV-2 infection after an exposure. Potential options currently under investigation in clinical trials include hydroxychloroquine, chloroquine, or lopinavir/ritonavir.
In general, adults patients with COVID-19 can be grouped into the following categories according to the illness severity.
| Category | Clinical features | Management guidelines |
| Asymptomatic/ Pre-symptomatic | Individuals who test positive for SARS-CoV-2 but have no symptoms. | Self-isolate at home.
No specific treatment. |
| Mild | Individuals who have any of the various signs and symptoms of COVID 19 (e.g., fever, cough, sore throat, malaise, headache, muscle pain) without shortness of breath, dyspnea, or abnormal chest imaging. | Should be closely monitored as the clinical course may rapidly progress.
Antiviral or immune-based therapy not recommended |
| Moderate | Individuals who have evidence of lower respiratory disease by clinical assessment or imaging and a saturation of oxygen (SpO2) ≥94% on room air at sea level. | Should be monitored closely given the possible risk of progression to severe illness in the second week after symptom onset.
Antibiotics, antiviral drugs and Immune based therapy |
| Severe | Individuals who have respiratory frequency >30 breaths per minute, SpO2 <94% on room air at sea level, ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300 mmHg, or lung infiltrates >50% | Oxygen therapy, empiric antibiotics, antiviral drugs and immune based therapy
Monitor for deveopment of complications |
| Critical | Individuals who have respiratory failure, septic shock, and/or multiple organ dysfunction. | Management of complications of COVID-19 and complications from prolonged hospitalization, including secondary bacterial infections, thrombo embolism, gastrointestinal bleeding, and critical illness polyneuropathy/myopathy. |
In pediatric patients, radiographic abnormalities are common and, should not be used as the sole criteria to define the illness category. Respiratory rate normal values also vary with age in children, thus hypoxia should be the primary criteria to define illness severity, especially in younger children.
Drugs under evaluation COVID-19 Management
No FDA-approved drugs have demonstrated safety and efficacy in randomized controlled trials when used to treat patients with COVID-19,
- Antiviral therapy: FDA has granted an Emergency Use Authorization for the use of remdesivir to treat severe cases. On June 15, The Food and Drug Administration revoked the emergency use authorization (EUA) that permitted the use of chloroquine and hydroxychloroquine to treat certain patients with COVID-19 due to potential toxicities.
- Dexamethasone: Based on large, multicenter, randomized trials, experts now recommends using dexamethasone (at a dose of 6 mg per day for up to 10 days) in patients with COVID-19 who are mechanically ventilated and in patients with COVID-19 who require supplemental oxygen but who are not mechanically ventilated.
- Immune based therapy: There are insufficient data to recommend either for or against the use of COVID-19 convalescent plasma or SARS-CoV-2 immune globulins, Interleukin inhibitors (Interleukin-1, Interleukin-6 inhibitors) and other immunomodulators (such as Interferons) for the treatment of COVID-19.
- Antithrombotic Therapy : Some patients with COVID-19 may develop signs of a hypercoagulable state and be at increased risk for venous and arterial thrombosis of large and small vessels.
|
Hypercoagulability and COVID-19 Pathogenesis: The pathogenesis for COVID-19-associated hypercoagulability remains unknown. However, hypoxia and systemic inflammation secondary to COVID-19 may lead to high levels of inflammatory cytokines and activation of the coagulation pathway. Laboratory abnormalities commonly include:
Manifestations: There are several reports of hospitalized patients with thrombotic complications, most frequently deep venous thrombosis and pulmonary embolism. Other reported manifestations include:
|
Pediatric Management
COVID-19 among pediatric patients is typically milder than among adults, with most children presenting with symptoms of upper respiratory infection. However, data suggest that infants (<12 months of age) may be at higher risk for severe illness from COVID-19 compared with older children.
Discontinuation of Transmission-Based Precautions or Home Isolation
Clinically recovered patients who are able to discharge from the hospital but who have not been cleared from their Transmission-Based Precautions may continue isolation at their place of residence until cleared.
Role of Nursing in COVID-19 Management
The WHO confirmed in May last year that 2020 would be dedicated to nurses and midwives. The nursing response to the coronavirus crisis has gone beyond the core purpose of 2020 as the International Year of Nurses and Midwives. The work done by nurses in the present crisis is a powerful and practical demonstration of nurses’ potential to address the great challenges of healthcare, which this year’s Nurses Day theme aims to achieve.
Nurses have proven to be warriors of health who have willingly taken responsibility with their hearts and souls. Nurses in all countries have “stepped up and gone beyond” their calling. They are working at the forefront and managing patient evaluations, placement and patient care in the COVID area. Nurses work 24 hours, do their best, and risk their lives, often with limited resources.
It is the nurses who have the responsibility and the accountability to provide reassurance to the patients and networking between between the needs of the patients and all the other departments of the hospital.
The entire nursing community is in the risk zone and we have all seen unprecedented levels of overwork by nurses, particularly those in intensive care units, those who are most directly involved in the response to the pandemic, often without an adequate rest, without support and assistance, with limited considerations for their mental health and well-being. However, these responsibilities and challenges are accepted voluntarily and happily by nurses.
The COVID-19 pandemic has brought nurses to the forefront of people’s minds and media attention, and their contribution to the prevention and promotion of healthcare is recognized more than ever.
Nurses are well aware of the challenges of providing high quality care in a time of pandemic and have demonstrated how they can improve productivity with sustained high quality. We salute these health warriors who work without expectation and sacrifice their personal and family lives and for their contribution in saving lives and improving health outcomes.
Covid 19 Nursing Management
NURSING ASSESSMENT
Nursing assessment of a suspected case should include:
- History including a detailed travel history: A detailed travel history for patients being evaluated with fever and acute respiratory illness should be obtained
- Assessment of signs and symptoms suggestive of COVID 19
- Assessment of risk factors of severe disease
Nursing Care Planning and Goals
1Impaired Gas Exchange
Goal
- The patient will maintain adequate ventilation.
Interventions
- Auscultate breath sounds as ordered
- Assess the respiratory rate, depth, and effort
- Perform chest physiotherapy as ordered, perform suctioning, as needed, and monitor response.
- Place the patient in High Fowler’s position that best facilitates chest expansion.
- Schedule care activities to allow uninterrupted periods of rest.
- Assist with activities of daily living.
- Administer prescribed medications, oxygen, as ordered, and monitor the patient’s response.
- Monitor arterial blood gas results and laboratory values.
- Monitor pulse oximetry as ordered
- Assist with intubation and mechanical ventilation as directed. Maintain ventilator settings, as ordered, and monitor the patient’s response.
2Hyperthermia related to infection and increase in metabolic rate.
Goals
- The patient will remain afebrile.
Interventions
- Monitor vital signs and body temperature as ordered.
- Provide high calorie easily digestible diet.
- Advice increased fluid intake.
- Provide tepid sponging and other non pharmacological measures to reduce hyperthermia.
- Administer antipyretic drugs as adviced.
3Risk for Infection related to failure to avoid pathogen secondary to exposure to COVID-19
Goals
- The patient will remain afebrile.
Interventions
- Auscultate breath sounds for changes in baseline.
- Monitor vital signs
- Review the signs and symptoms of infection with the patient; discuss preventive methods to reduce the risk of infection.
- Instruct the patient and family to use disposable gloves and household disinfectant to clean any surfaces that may have been exposed to the patient’s body fluids.
- Administer prescribed medications, and monitor for effect.
- Maintain infection control precautions according to recommendations.
- Monitor laboratory values.
4Impaired Comfort
Goals
- The patient will verbalize or demonstrate feelings of comfort.
Interventions
- Assess the degree of discomfort of the patient (characteristics, severity, location, onset, type, precipitating factors and duration).
- Assess for nonverbal cues of discomfort, such as restlessness, muscle tension, or altered vital signs.
- Educate the patient on ways to decrease the factors that precipitate discomfort, as appropriate.
- Provide non-pharmacologic comfort measures. Adjust the environment, as necessary, and position the patient for comfort.
- Administer prescribed medications, and monitor for effect.
5Activity Intolerance
Goals
- The patient will exhibit tolerance of increased physical activity.
Interventions
- Assess the client’s ability to perform activities of daily living.
- Assist the patient in prioritizing activities.
- Encourage alternating periods of rest and activity.
- Encourage nutritional intake.
- Provide assistance with care activities, as indicated.
- Provide supplemental oxygen, as ordered, and monitor the patient’s response.
- Monitor vital signs during and after activity.
6Altered Skin Integrity Risk
Goals
- The patient will maintain intact skin.
Interventions
- Assess skin, noting color, moisture, texture, temperature; note erythema, edema, and tenderness. Assess the skin over bony prominences.
- Assess the patient for fecal and urinary incontinence.
- Assess nutritional status; assist the patient in choosing nutrient-rich foods to promote health and wellbeing.
- Encourage mobility. Assist the patient in getting out of bed, as needed.
- Reposition the patient at an interval determined by the patient’s tissue tolerance, level of activity and mobility, skin condition, overall medical condition, treatment goals, support surface in use (if applicable), and comfort.
- Pad bony prominences and other vulnerable areas, as indicated.
- Use measures to prevent or such as a pressure-reducing mattress or overlay.
7Anxiety related to unknown etiology of the disease.
Goals
- The patient will verbalize anxiety, concerns, and fears.
Interventions
- Assess for signs and symptoms of anxiety.
- Explain all tests and procedures using simple and clear explanations.
- Teach stress-reduction and relaxation techniques
- Provide care in a calm and reassuring manner.
- Administer prescribed medications, and monitor for effect.
8Knowledge Deficiency related to unfamiliarity with disease condition
Goals
- The patient will demonstrate knowledge retention related to COVID-19.
Interventions
- Assess the patient’s existing knowledge level and barriers to learning.
- Establish realistic goals for learning.
- Provide necessary information regarding the disease, its management, complications that can arise,diagnostic testing, prevention of disease transmission to family and others.
- Tailor the teaching to the patient’s individual communication, learning, and cultural needs.
- Evaluate the patient’s understanding of the topics discussed.
- Involve the patient’s family or caregivers in teaching (as appropriate).
9Malnutrition
Goals
- The patient will achieve an adequate daily caloric intake.
- The patient will maintain an adequate weight.
Interventions
- Assess the patient’s nutritional intake per meal and per 24 hours.
- Assess and monitor laboratory values.
- Assist with dietary choices.
- Assist the patient with meals, as necessary.
- If administering tube feeding, monitor gastrointestinal status and residuals, as ordered.
- Monitor weight daily or as ordered.
- Administer parenteral nutrition, as ordered, and monitor the patient’s response.
10Psychosocial and Spiritual Needs
Goals
- The patient will express decreased feelings of guilt and fear.
Interventions
- Develop an honest, trusting, and open relationship with the patient.
- Provide support to the patient and family (as appropriate).
- Provide a calm, peaceful environment. Discuss the use of meditation or relaxation activities.
- Show your acceptance of the importance of the patient’s religious beliefs and practices.
- Collaborate with a psychologist, as necessary.
References
- National Institutes of Health. COVID-19 Treatment Guidelines Panel. Corona virus Disease 2019 (COVID-19). Treatment Guidelines. Available at https://www.covid19treatmentguidelines.nih.gov/. Accessed June 28, 2020.
- World Health Organization. Coronavirus disease (COVID-2019) situation reports. 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/. Accessed April 9, 2020
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): cases in U.S. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed April 9, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): People who are at higher risk for severe illness. 2020. Available at:https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html. Accessed April 8, 2020.
- Mason R J. Pathogenesis of COVID-19 from a cell biology perspective. European Respiratory Journal 2020 55: 2000607; DOI:1183/13993003.00607-2020
- Ministry of Health and Family Welfare. Directorate General of Health Services Goverment of India.2020. Clinical management protocol: Covid-19 [PDF file]. Retrieved from https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19.pdf.
- Lippincott Advisor Nursing Care Plans for Medical Diagnoses. Coronavirus disease 2019. Retrieved fromhttps://www.nursingcenter.com/nursingcenter_redesign/media/nursingcenter/coronavirus%20files%20from%20Lippincott%20Solutions/%C2%A9-2020-Lippincott-Advisor-Nursing-Care-Plans-for-Medical-Diagnoses_-Coronavirus-disease-2019-(COVID-19).pdf.









thank you the whole information is so organized. Thanks
A very concise topic👍. Keep it up! How to gain a certificate for this topic?
Nice topicc
How can I get the certificate for this with CPD? Thank you
Please check this article https://rnspeak.com/free-cpd-for-nurses/
Thank you for sharing. Looking forward to more inputs
Thank u
Welcome 😉
Welcome!
Welcome Teresa! Our goal is to provide a good content for our nursing colleague
Great read! This article really gave me more knowledge about COVID-19. It’s so good to know this. Thanks for sharing this information!
Welcome 🙂